
The majority of the NCEs in the pipeline, especially in the case of oncology and anti-infective indications, are poorly soluble and have posed a major threat to the overall drug development process.
INTRODUCTION
The term solubility is quantitatively referred to as the amount of solute dissolved in a specified quantity of solvent at a definite temperature and preferably stated in either of the expressions viz. parts, percentage, molality, molarity, volume fraction, or mole fraction. Qualitatively, solubility confers to the spontaneous collaboration between two substances to create a homogenous dispersion at the molecular levels, where a solute is in equilibrium with the solvent in a saturated solution. The poorly soluble drugs are s practically less bioavailable upon oral administration, which hold back their biological performance and clinical response (therapeutic efficacy). The other factors those shackles the bioavailability of APIs includes the slow dissolution, poor permeability, high p-glycoprotein efflux, high extent of first-pass metabolism, degradation of APIs in gastrointestinal tract, and equally important is their inappropriate partition coefficient.1
In the above context, the Biopharmaceutics Classification System (BCS) provides a framework for relating oral drug absorption to gastric permeability and solubility. Commercial drug products exhibit a fairly uniform distribution between the drug classifications, with the Class I (highly soluble, highly permeable) candidates being the top ranked. However, they are around 5-10% of the new chemical entities (NCEs) in the pipeline whereas around 90% NCEs belongs to the Class II & IV, less soluble and less permeable, respectively (Figure 1). As stated earlier, their performance and clinical response is questionable upon oral administration. The United States Government Accountability Office reported a 10,000:1 attrition rate for NCEs from early-stage drug discovery to clinical trials, and then to federal review and approval. This resulted in tremendous fall in R & D productivity teamed with massive translational costs in the current pharmaceutical space.2
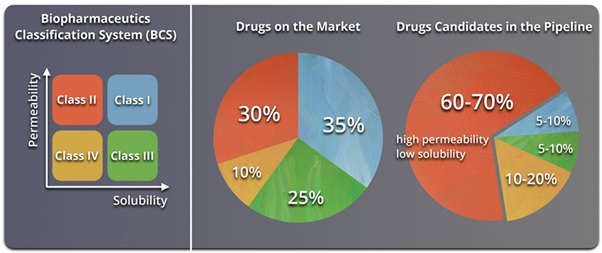
Figure 1. Solubility challenges that plague the oral drug-delivery frontier.2
To tackle the issue of poor solubility and bioavailability, a variety of technologies have been adopted by the scientists around the globe. The particle technologies intended for improving the drugs’ solubility and bioavailability include the mechanical micronization and/or nanonization (jet milling, ball milling, and high-pressure homogenization), cryogenic spraying or crystal engineering for precisely controlling the particle size of the APIs, and formulating solid self-emulsifying drug deliveries, polymeric micelles, lyophilized liposomes, solid lipid nanoparticles and inclusion complexation of APIs with cyclodextrins or cyclodextrin-derivative(Figure 2).
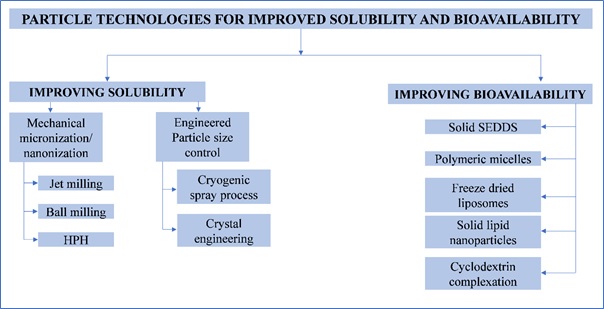
Figure 2. Particle technologies for improving drugs’ solubility and bioavailability.
NANOSUSPENSIONS
Introduction to nanosuspensions:
Pharmaceutical nanosuspension is a very finely dispersed solid drug particles in aqueous or non-aqueous vehicle which are stabilized by surfactants and size distribution usually remains less than one micron. These are used for delivering the APIs via different routes of administration viz. oral, parenteral, ocular, pulmonary, topical, and so on. Additionally, pharmaceutical nanosuspensions are capable of surface functionalization to achieve target-specific and controlled delivery. If appropriately formulated, one can achieve a long-term stability of nanosuspensions for 24-36 months, either at room- or refrigeration temperatures. Despite their beneficial traits, the nanosized suspensions suffers few major issues viz. sedimentation may occur if not stored properly, and difficulty in delivering an accurate and/or uniform dose. Handling and logistics issues sometimes creates problems prior to reaching the end-user.3
Nanosuspensions are composed of dispersed solid particles of ~200-600 nm size. Maintaining a crystalline state leads to an increased dissolution rate and enhanced bioavailability in nanosuspensions. The major reason behind increased solubility involves the particle size reduction through one of the following major mechanisms viz. cleavage, fracture, abrasion, chipping, or shattering, resulting in increased surface area. This leads to high exposure of the size-reduced API particles to the solvent molecules, resulting in ultimate enhanced solubility and bioavailability.
The formulation components of nanosuspensions includes stabilizers, surfactants, osmogens, and cryoprotectants. Generally, the pharmaceutical nanosuspensions are fabricated in variety of dosage forms viz. liquid, lyophilized powder, or incorporated into dry pellets, tablets or minitablets, capsules, and films (Figure 3).
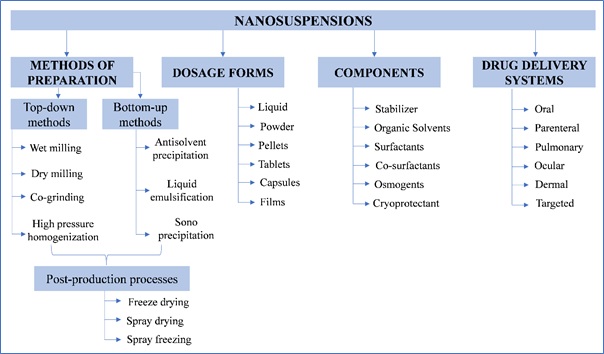
Figure 3: Methods of preparation, different suspension dosage forms, components, and drug delivery systems based on suspensions.
Approaches of nanosuspension formulation
There are numerous options to produce nanosuspensions. Basically, these approaches are categorized as top-down approaches (wet milling, dry milling, co-grinding, and high-pressure homogenization) and bottom-up approaches (antisolvent precipitation, sono-precipitation, and liquid emulsification). Top-down approaches are typically based on the principle of mechanical attrition to render large crystalline particles into nanosized ones, while Bottom-up technology involves dissolving the drug in a solvent and precipitating it in a controlled manner to nanoparticles through addition of an anti-solvent in the presence of surfactant. 3
Some of the popular technologies successfully prepared using Top-down approach (particle size reduction by mechanical means) are NanoCrystal® wet-milling technology (Elan Corporation, Ireland) and Dissocubes® high-pressure homogenization technology (Drug Delivery Services GmbH, Germany), while those prepared using Bottom-up approach (controlled precipitation/ crystallization) has been explored by Dow Pharma (USA) and BASF Pharma Solutions (USA). NanoEdge® technology (Baxter International Inc., USA) used a hybrid technology by employing both top-down and bottom-up approaches to fabricate nanosuspensions through microprecipitation and homogenization techniques.4
The schematic diagram illustrating the fabrication of nanosized suspensions using top-down and bottom-up approaches is illustrated in Figure 4.
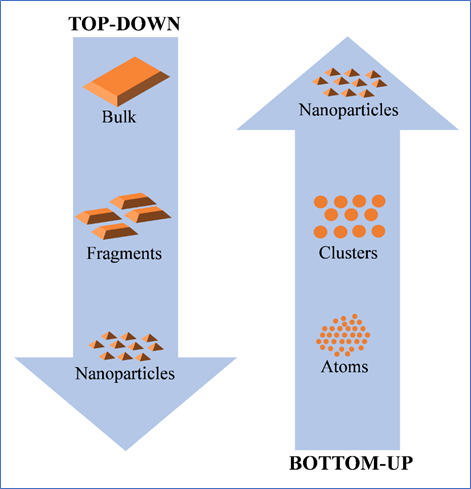
Figure 4: A schematic illustration hypothesizing the formation of nanosized particles for dispersion via top-down and bottom-up approaches.
Stability issues in nanosuspensions and remedies therein
Despite the vast progress in the field of nanosizing the APIs to develop stable pharmaceutical nanosuspensions, the nucleation and particle growth of the dispersed particles triggered at the molecular levels impede the stability of nanosuspensions. The high surface area of drug nanosuspensions, by the virtue of which they exhibit their unique biopharmaceutical characteristics, also renders them thermodynamically unstable and thereby promotes the variety of instability problems viz. Ostwald’s ripening, agglomeration, and crystal growth. These stability issues are the inevitable encountered problems in the development of nanosuspensions technology and in exploring their pharmaceutical applications at industrial levels.5
The change of Gibb’s free energy leads to the enhanced particle mobility and this results into the formation of thermodynamically unstable nanosuspensions. This phenomenon is responsible for Ostwald’s ripening, agglomeration, and crystal growth in nanosuspension. To overcome these issues, selection of an appropriate stabilizer is essential that can alter the surface of dispersed drug particles in nanosuspensions and stabilize them through either steric or electrostatic means. Stabilizers are vital components which are generally used to prevent the aggregation of high-energy nanosuspensions.5
The transition of size ranges, either from molecular dispersions to colloids or from colloids to coarse dispersions, is very gradual. However, when the gravity of the dispersed phase drug particle is greater than the buoyancy force of dispersion system provided, results in sedimentation.5
Flocculation, a type of sedimentation, is an out of equilibrium phenomenon of a colloidal nanosuspension. And it results from attractive interactions between particles, and its kinetics can be quantitatively related to the so-called DLVO potential. Flocculation of nanosuspensions may occur by polymer bridging, charge neutralization, polymer-particle surface complex formation or depletion flocculation, or by a combination of these mechanisms. There are two main possible mechanisms of flocculation: (i) bridging due to the adsorption of a macromolecule onto the surface of nanocrystals and (ii) surface charge neutralization.5
The aggregation of dispersed phase particles, which leads further to sedimentation, and instability of nanosuspension, is exhibited through any of the three mechanisms viz. (i) perikinetic aggregation (aggregation induced by Brownian motion), (ii) differential sedimentation (aggregation induced by particle of different size and density), and (iii) orthokinetic sedimentation (aggregation induced by shear forces of stirring or flow).
Practical considerations for stabilizer selection
Selection of an appropriate stabilizer is a crucial step in the formulation of stable nanosuspension. Many researchers have extensively studied this aspect and established a relationship between stabilizer efficiency and their properties. These properties of stabilizers influencing the efficiency of stabilizer includes (i) drug-related parameters, (ii) stabilizer-related parameters, and (iii) dispersion medium-related parameters. The practical considerations for selection of an appropriate stabilizer are presented in Figure 5.
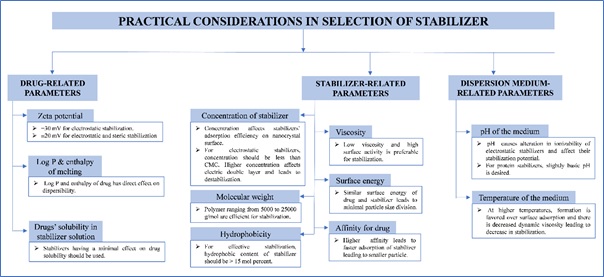
Figure 5: Practical considerations in selection of stabilizers for nanosuspension formulation.
CASE STUDY
Recently, Na et al. (2020) used a nanosuspension approach to enhance the solubility and oral bioavailability of ticagrelor (a Class IV drug and reversible P2Y12 receptor antagonist used in the treatment of cardiovascular diseases like stroke). D-α-tocopherol polyethylene glycol 1000 succinate (TPGS) and polyvinyl alcohol (PVA) have been used as stabilizers in appropriate concentrations to produce nanosuspensions using an antisolvent precipitation method (a kind of Bottom-up approach). The dispersed phase size was around 206 nm whereas pharmacokinetic studies (in male Sprague-Dawley rat model) have demonstrated a significant rise in the oral bioavailability (2.2-fold) of ticagrelor as nanosuspension compared to commercial product, Brilinta®. Authors concluded that the enhanced solubility and permeation across the GI membrane had a synergistic effect on the oral absorption.
CONCLUSION ANF FUTURE OUTLOOK
The field of nanomedicine has emerged with several promising approaches for the delivery of therapeutics utilizing the benefits of the nanoscale carriers. One of the nanotechnology-based approaches is the formulation of nanosuspension and is considered as the most favorable delivery system for poorly soluble drugs, due to less inter- and intra-subjects’ variances and high systemic availability.
Nanosuspensions altered the pharmacokinetic performance of variety of drugs, thereby improved the safety and efficacy. So, following the biological assessment in suitable animal model, the establishment of an in vitro/in vivo correlation will become an attractive research area in the further study of nanosuspensions.
Additionally, their applications in buccal, nasal, and topical delivery will represent a more beautiful prospective. Surface modification of the drug nanosuspensions can further increase the benefits viz. stabilizing blood level of drugs by controlling drug release and targeting specific organ using surface ligands, which further elicit the active targeting, would be regarded as the much encouraging step in the development of cost-effective and efficient nanosuspension in near future.
Abbreviations: NCEs: New chemical entities; PK: Pharmacokinetics; API: Active pharmaceutical ingredients; BCS: Biopharmaceutical classification system; R&D: Research and Development; TPGS: D-α-tocopherol polyethylene glycol 1000 succinate, PVA: Polyvinyl alcohol].
REFRENCES